What is gadolinium?
In the health care field, gadolinium-based contrast agents (GBCAs) have been used extensively as a chemical contrast dye since 1988. They are important in neutron radiography, particularly in their use in in targeting tumors in neuron therapy, enhancing magnetic resonance imaging (MRI), and in the diagnosis and treatment of cancer. Gadolinium is actually a chemical element found in the earth’s crust – called a rare earth element – that is vital to many modern technologies. Its compounds are used to make magnets, electronic components and data storage disks, are used as green phosphors in color TV picture tubes, and as shielding in nuclear reactors. You may be asking “Is Gadolinium safe to put in my body?”.
Is Gadolinium safe?
Gadolinium’s fluorescent properties are what makes it a major contributor in modern healthcare imaging, particularly MRI and MRA technology. It aids in visualizing body tissue that cannot always been seen without this contrast material.
Unfortunately, like all heavy metals, gadolinium ions (charged particles) in certain forms are toxic to mammals. It can cause inflammation, neurological damage, oxidative stress, and can be damaging to DNA. For that reason, other compounds must be used when it is injected into the body in order to modify the toxic effects (called chelation) until the substance can be excreted from the body.
In some cases, the patient will not feel any after-effects of gadolinium being injected into the body and it can presumably be flushed out of the body thanks to healthy kidney function; but this is certainly not always what occurs. For those with impaired kidney function, injecting these metals into the body can actually be life threatening.
Researchers are finding that even in those with healthy kidney function, however, the after-effects of gadolinium can remain and be lasting. Additionally, it appears that it may not be “flushed out” from the body in a matter of hours as was previously believed. According to Rogosnitzky and Branch (2016), one study found gadolinium deposits in the bone of hip replacement patients eight years after exposure. In fact, the FDA has recognized that repeated use of gadolinium can result in deposits in the brain and other tissues; for that reason, it is now recommended that its use be limited.
Gadolinium and Adverse Events Reported to the FDA
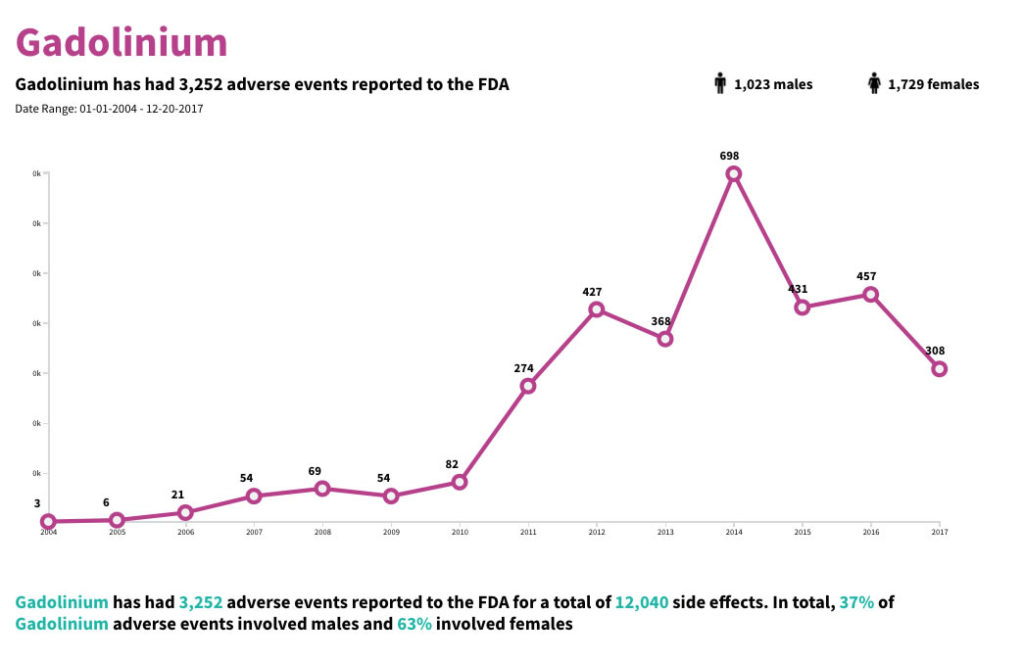
There have been over 3,000 Gadolinium adverse events reported to the FDA. Over 12,000 side effects have also been reported from 2004-2017.
More serious or allergic reactions can include:
- Swelling of face or tongue
- Difficulty breathing
- Rash
- Confusion
- Seizure
- Confusion
- Mood changes
- Coma
- Urinary problems (incontinence, urgency)
- Increased thirst
- Swelling
Even “normal” side effects can be uncomfortable. While not considered serious, these can include:
- Headache
- Nausea
- Dizziness
- Hot flash/flushing
- Burning/pain at injection site
- Unpleasant/metallic taste in mouth
- A feeling of cold or clamminess
What happens when gadolinium is injected into the body?
Gadolinium is injected into the body intravenously (into a vein) and then travels throughout the entire body, including the brain. It can then be deposited in almost any tissue in the body.
Historically, chelated gadolinium has been thought to be quickly cleared from the body via excretion (as long as the kidneys are functioning properly). Studies in both animals and humans have revealed that this is not the case, however, especially when the substance is given on multiple occasions. Gadolinium was found to leave deposits in the skin, bone, and other areas of the body, regardless of the quality of kidney function. Because it crosses the blood brain barrier, deposits can be found in the brain as well. Whether or to what extent any adverse health effects result from such deposits – even in the absence of symptoms – is still under investigation.
What are the possible effects of gadolinium toxicity?
Gadolinium toxicity can come in various forms, as those who have experienced such side effects have reported. Such effects can include headache, bone and joint pain, a feeling of burning or pins and needles under the skin, and more (Report: Team of Patient Advocates, 2013).
The first reports of an actually fatal gadolinium toxicity disorder were published in 2006. One of the first serious side effects of gadolinium administration was found when nephrologists connected GBCA administration to a disease called nephrogenic systemic fibrosis (NSF). NSF affects the skin and internal organs or those exposed to gadolinium and was seen in patients with poor kidney function.
NSF symptoms include the following and may appear within days or months after a gadolinium-dye injection:
- Pain deep in your hip bones or ribs
- Swelling, hardening and tightening of your skin
- Reddened or darkened patches on the skin
- Burning or itching of your skin
- Yellow raised spots on the whites of your eyes
- Limited mobility due to stiffness of joints
- Muscle weakness
As a result, manufacturers were told by the FDA in 2007 to include a black box warning on their packaging. Black Box Warnings are the strictest warnings found on a drug or product packaging and indicate reasonable evidence of an association of a serious hazard with the drug. At that point in time, it was believed that only patients with kidney damage or kidney disease were in danger of the effects of gadolinium toxicity, but history has proven otherwise.
In 2009, a review by medical experts found a link between GBCAs and Nephrogenic Systemic Fibrosis, warning that gadolinium was inappropriate for use in patients with kidney disease. In 2010, the FDA issued a second news release announcing that new warnings were required on the use of gadolinium based agents and that enhanced screening of patients was recommended to detect kidney dysfunction before their use. Additionally, reports of long-term accumulation of gadolinium in patients with normal kidney function began to appear in the medical literature.
In 2014, a study by Kanda et al. revealed that deposits were found in the brains of patients with normal kidney function who had been repeatedly exposed to gadolinium.
In 2015, the FDA published a safety announcement regarding its investigation of the repeated use of gadolinium substances in MRI and the risk of long-term deposits on the brain.
A 2016 study pointed to gadolinium contamination in the San Francisco Bay water near one of the medical centers there, citing concern over the incredible increases of the substance in the waters there. The Environmental Science Technology report found that gadolinium levels increased significantly in water samples collected from 1993 to 2013. “In the southern part of the bay, which is surrounded by medical and industrial centers and receives their wastewater, all of the elements showed increases over the time period studied. Gadolinium in particular increased from 23.2 pmol/kg in 1993 to 171.4 pmol/kg in 2013” (Kemsley, 2016, para.2).
Researchers are now looking for possible delayed or chronic signs of gadolinium toxicity in patients, particularly those who have had multiple exposures to it. According to the FDA’s Adverse Events Reporting System (FAERS), 886 cases and 2,317 adverse events have been reported since 2006: 799 of those were serious and included 187 deaths.
While infrequent, case reports of patients experiencing kidney and liver damage, pancreatitis, reduced white blood cell count, abdominal pain and vomiting, inflammation, and other adverse effects after dosing have occurred. As it is now known that gadolinium is not completely excreted from the body as was previously believed, multiple or recurrent dosing has now been shown to be of particular concern as there is an accumulation effect of this substance.
What Companies Make Gadolinium?
GBCAs have a variety of trade names that have been used over the years, including the following:
| Trade Name | Approved |
| Magnevist | 1988 |
| ProHance | 1992 |
| Omniscan | 1993 |
| OptiMARK | 1999 |
| MultiHance | 2004 |
| Eovist/Primovist | 2008 |
| Ablavar/Vasovist | 2008 |
| Gadavist | 2011 |
| Dotarem | 2013 |
Gadolinium Deposition Disease
A landmark research article released in 2016 by Semelka et al. proposed the name Gadolinium Deposition Disease for a disease process seen in patients who had normal or near normal function of the kidney but who still developed persistent symptoms of gadolinium toxicity hours to months after these contrast agents were used on them.
Retention of gadolinium in the human body is known to have serious consequences, including a variety of side effects as well as an incurable and potentially life-threatening disease known as NSF. While GBCAs and their full and long-term effects on the body are still being investigated, they are still being routinely used on patients. The bottom line is that gadolinium poses a potential risk to all patients and greater awareness is needed.
Latest FDA update
At the end of 2017, the latest warning update by the FDA was released. On December 19, 2017 an FDA update to the May 2017 communication now requiring a new class warning as well as other safety measures for all GBCAs used in MRI. Recommendations stated that health care professionals and patients should carefully consider the retention characteristics of these drugs. Gadolinium-based drugs can remain in all parts of the body, including the brain, for months to years after receiving them. The FDA communication was addressed to radiologists, health care professionals, and patients alike.
Current lawsuits
Gadolinium deposition lawsuits against the makers of these toxic contrast agents are now being filed in both federal and state courts. As the results of the toxic effects of these agents are beginning to come to the forefront, patients who are alleging that they were not properly warned about the risks of these products are demanding accountability from manufacturers of GBCAs. Actor Chuck Norris’ wife is one of these, and in a compelling article, Norris confronts the federal government on its silence on this issue and encourages greater awareness about the dangers of gadolinium use.
Manufacturers of gadolinium currently involved in lawsuits or potential lawsuits include the following:
- Bayer HealthCare Pharmaceuticals (Magnevist)
- GE Healthcare (Omniscan)
- Tyco Healthcare (Optimark)
- Bracco Diagnostics Products (Prohance, Multihance)
The patients and families of those who were diagnosed with or died from NSF after receiving GBCAs made by Magnevist, Omniscan, or OptiMARK have filed lawsuits against these manufacturers. While some lawsuits have been settled, while others are still pending.
Can I be tested for gadolinium toxicity?
Testing for the presence of gadolinium is not yet an established practice, so currently, there is no medical provider or institution that can definitively determine whether your symptoms are a result of gadolinium toxicity. You still can, however, have your urine and blood tested, and even skin biopsies may be used to test for the presence of gadolinium, but since it tends to deposit in bone and not remain in circulation, it is likely that results of such tests will be negative.
How to report an adverse event from a GBCA
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.fda.gov/MedWatch/report
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178